Researchers reveal gene that suppresses growth of colorectal cancer tumors
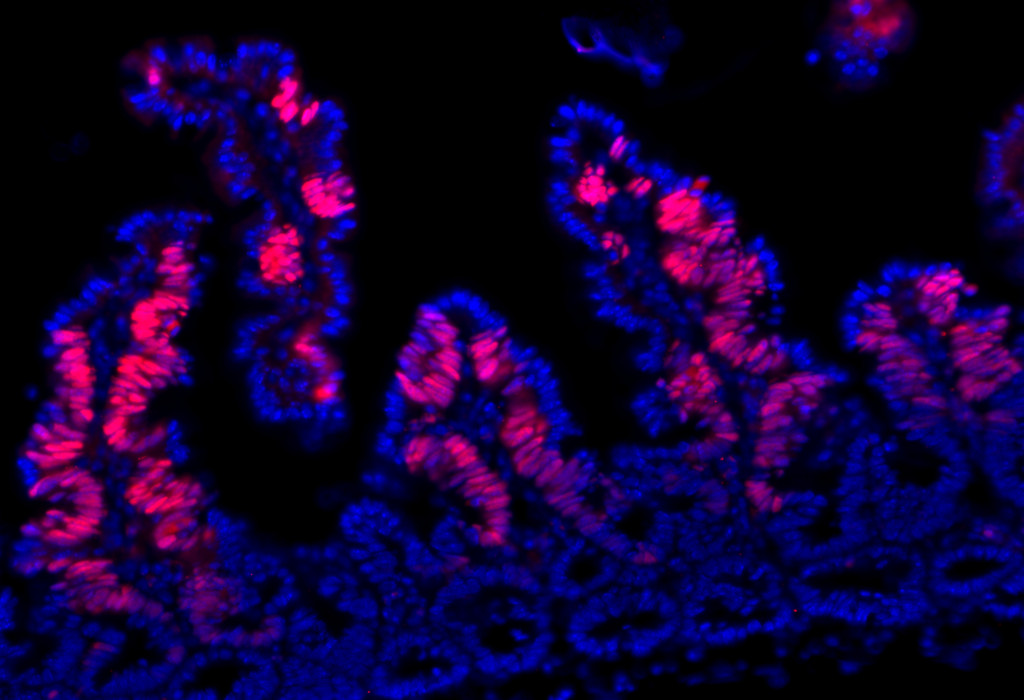
Cancer stem cells can reside in niches, which are distinct regions within the tumor microenvironment. Stem cell niches (dyed pink) and nuclear DNA (blue) of a gut adenoma.
The new research findings help to understand mechanisms that lead to different types of intestinal tumors. They show that the Lef1 gene suppresses the development and growth of colorectal cancer by restricting the formation of cancer stem cell niches.
In the recent issue of Science Advances, cancer researchers from the iCAN Digital Precision Cancer Medicine Flagship at the University of Helsinki and HUS Helsinki University Hospital report novel research findings on stem cells in benign tumors of the gut, in which colorectal cancer originates. They demonstrate that the Lef1 gene suppresses the development and growth of cancer by restricting the formation of cancer stem cell niches. The Lef1 gene is induced in tumors known as adenomas in the gut.
Lef1 gene restricts formation of cancer stem cell niches
What makes the report noteworthy is that cancer stem cells are important targets in the treatment of cancer. Cancer stem cell niches can support stem cell growth as distinct regions within the tumor microenvironment. The investigators found that when expression of the Lef1 gene was blocked, tumor stem cell niches increased, and tumor growth was markedly accelerated.
“The Lef1 gene was discovered 30 years ago, and 20 years ago its function was linked to colorectal cancer. It was assumed that Lef1 functions like its relative, the Tcf4 gene, by increasing cell growth in healthy intestine and benign polyps and adenomas”, says Academician Kari Alitalo, University of Helsinki, the corresponding author of the study.
The new results by the team now show the opposite. Unlike its relative genes, the Lef1 gene is not expressed in healthy intestinal stem cell crypts but is activated in the precursor cells that develop into intestinal cancer.
Researchers also found that the human Lef1 gene is not active in so-called serrated cancers of the large intestine, referring to the serrated pattern on the surface of the tumor formed by proliferating stem cell crypts.
Results may help to find new treatment targets
The new research findings help to understand the mechanisms that lead to different types of intestinal tumors. The results may also help to identify new treatment targets. The function of the Lef1 gene in the body is to regulate the activity of hierarchically downstream genes. It is amongst these genes that future research may find new treatment targets for blocking of cancer stem cell niches.
Cancer stem cell-targeted therapies play a key role in the treatment of cancer because other, more differentiated cells in the tumors have a relatively short lifespan even without treatment. In the normal intestine, most differentiated cells, such as those that produce mucus or intestinal hormones or transfer foodstuffs to the body, regenerate from stem cells according to a complex genetic program in less than a week. Meanwhile, “old” intestinal cells are eliminated in the feces.
This genetic program is disrupted when DNA damage activates a cancer gene or inactivates a gene that restricts cell growth. This means that the mutant cells remain locked to further growth, making them vulnerable to mutations in other genes needed for their development and growth into a malignant cancer. This is usually a slow process that takes years. It is therefore advisable to remove precursors of intestinal cancer during intestinal endoscopy, especially in the case of elderly persons.
Photo: Cancer stem cells can reside in niches, which are distinct regions within the tumor microenvironment. Stem cell niches (dyed pink) and nuclear DNA (blue) of a gut adenoma.
Article: Sarika Heino1, Shentong Fang1,2, Marianne Lähde1, Jenny Högström1, Sina Nassiri3, Andrew Campbell4,5, Dustin Flanagan4,5, Alexander Raven4,5, Michael Hodder4,5, Nadia Nasreddin6, Hai-Hui Xue7, Mauro Delorenzi3,8,9, Simon Leedham6, Tatiana Petrova8,9, Owen Sansom4,5, Kari Alitalo1*. Lef1 restricts ectopic crypt formation and tumor cell growth in intestinal adenomas, Science Advances. DOI: 10.1126/sciadv.abj0512
1) Translational Cancer Medicine Program (CAN-PRO), iCAN Digital Precision Cancer Medicine Flagship and Wihuri Research Institute, Faculty of Medicine, HiLIFE-Helsinki
Institute of Life Science, Biomedicum Helsinki, University of Helsinki, 00014 Helsinki,
Finland. 2) School of Biopharmacy, China Pharmaceutical University, Nanjing
211198, P.R. China. 3) Bioinformatics Core Facility, SIB Swiss Institute of Bioinformatics,
Lausanne, Switzerland. 4) Cancer Research UK Beatson Institute, Garscube Estate,
Glasgow G61 1BD, UK. 5) Institute of Cancer Sciences, Garscube Estate, Glasgow G61
1QH, UK. 6) Intestinal Stem Cell Biology Laboratory, Wellcome Trust Centre for Human
Genetics, University of Oxford, Oxford OX3 7BN, UK. 7) Center for Discovery and
Innovation, Hackensack University Medical Center, Nutley, NJ 07110, USA. 8) Department
of Oncology, University of Lausanne and CHUV, Epalinges, Switzerland. 9) Ludwig
Institute for Cancer Research Lausanne, Epalinges, Switzerland.
*Corresponding author.
iCAN is a national research flagship focusing on digital precision cancer medicine. Through multidisciplinary research across cancer types, iCAN aims to create breakthrough discoveries for advancing personalized cancer diagnosis and treatment. iCAN is hosted by the University of Helsinki and HUS Helsinki University Hospital.
Further information
Kari Alitalo, Academician, University of Helsinki
kari.alitalo@helsinki.fi
+358505003572
Sarika Heino, FM, Researcher, University of Helsinki
sarika.heino@helsinki.fi